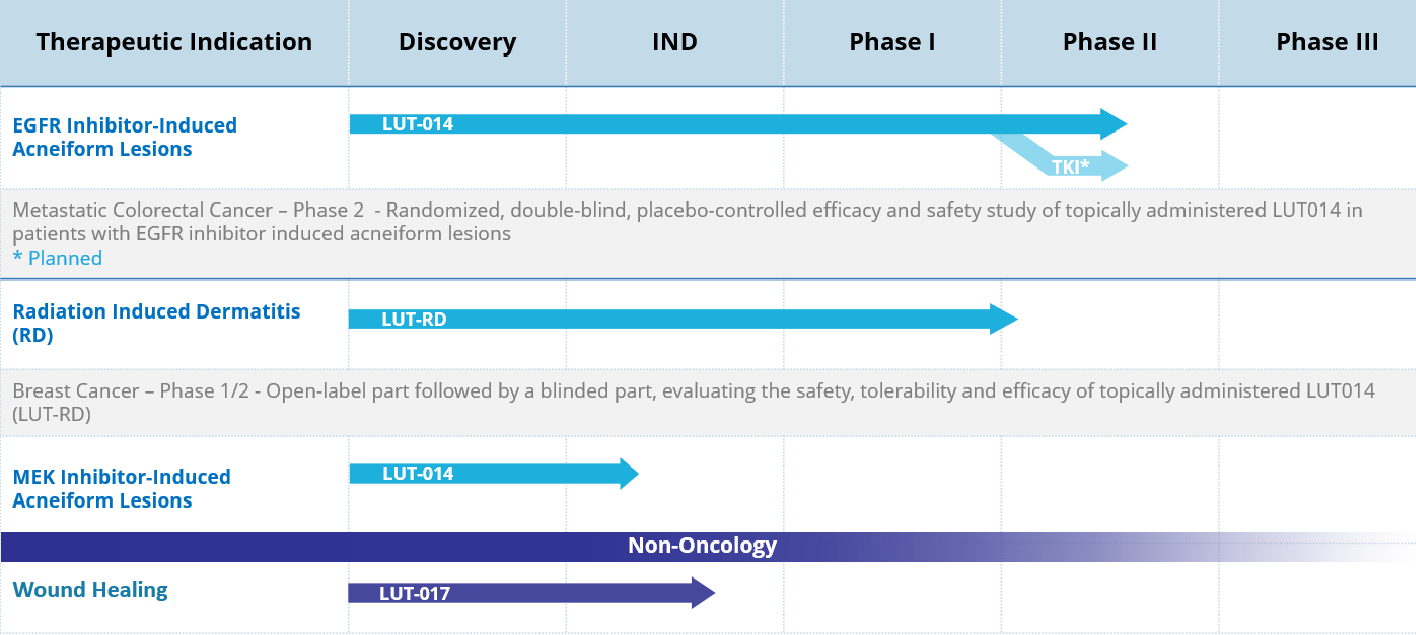
Following successful completion of a phase 1 study, Lutris Pharma is conducting a phase 2, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topically administered LUT014 in metastatic colorectal cancer patients with EGFR Inhibitor Induced acneiform lesions. The phase 1 study demonstrated the first evidence of treatment effectiveness by reducing the severity of acneiform lesions and improving patient quality of life. Promising preliminary data from the phase 2 study may point to a dose response and the high dose success rate achieved demonstrates efficacy or LUT014. The hypothesis is that administration of B-Raf inhibitor LUT014 topically to a regimen of EGFR inhibitors in these patients, reversed its inhibitory effect on downstream proteins in the skin cells and decreased the associated acneiform lesions induced by the EGFR inhibitors.
Lutris Pharma successfully completed a phase 1/2 study to assess the efficacy, safety and tolerability of topically administered LUT014 for the treatment of radiation-induced dermatitis in breast cancer patients. Positive top-line results from the open label part 1 and double blinded part 2 of the study were reported in September 2022, showing that 75% of patients in part 1 experienced complete resolution of radiation dermatitis and 25% of patient experienced improvement in grading, while part 2 data showed a clinical treatment effect of 28% when comparing patients on LUT014 to patients on Placebo.