New investigation gel (LUT014) for the treatment of acneiform lesions in metastatic colorectal patients – now open to enrolling patients
WHAT ARE ACNEIFORM LESIONS?
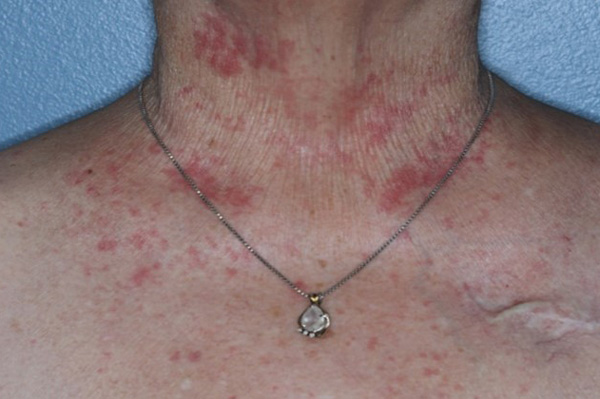
Acneiform lesions are a common side effect of a specific type of anti-cancer therapy with an EGFR inhibitor given for colorectal cancer. These lesions are an “acne-like” rash and appear primarily on the face, scalp, chest, and upper back.
WHAT ARE THE SIGNS AND SYMPTOMS OF ACNEIFORM LESIONS?
Up to 80% of the patients being treated with EGFR inhibitors, develop an acneiform rash at some grade. Symptoms usually develop in the first 1-2 weeks after initiation of the EGFRI therapy. The rash generally consists of a form of skin eruptions that are painful and tender to touch and eventually transition to crusts.
WHAT CAN BE DONE TO REDUCE THE SEVERETIY ACNEIFORM LESIONS?
Currently, no drug or treatment is approved by the US Food and Drug Administration (FDA) for the prevention or treatment of EGFR Inhibitor induced acneiform lesions.
LUT014 GEL: A NEW INVESTIGATIONAL TREATMENT FOR ACNEIFORM LESIONS
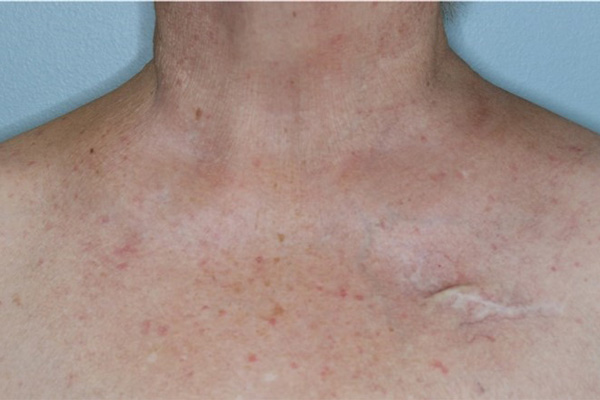
LUT014 is an investigational drug applied topically as a gel that is being tested to see if it will reduce the severity of EGFRI-induced acneiform lesions.
Pictures of baseline and on-therapy areas of rash in patient from phase 1 study after 1 week of treatment with LUT014